Water Quality Technical Note - Iron (Fe)
Iron (Fe) is an essential micronutrient necessary for plant growth, but when present at high concentrations in agricultural water it can damage irrigation infrastructure, increase system maintenance, and inhibit plant productivity. In California’s North Bay Area, iron commonly enters irrigation water through groundwater and, in some cases, surface waters with anaerobic (low-oxygen) conditions. Understanding the sources, behavior, and management of iron is crucial for optimizing farm water management and protecting plant health.
Sources of Iron in Irrigation Water
Iron occurs naturally in soils and rocks across the globe, including serpentine and other soils found in Northern California, but concentrations can differ dramatically even at small spatial scales. From soils, iron can enter water supplies through dissolution and erosion, microbial redox reactions, and anthropogenic sources such as industrial wastewater discharge. In the North San Francisco Bay and Northern Coast regions, iron in groundwater is common, and has been recorded at high concentrations in many areas of the regions subbasins.
Soluble iron is mobile in water and most commonly found in groundwater but can also be present in low oxygen surface water. Under anaerobic conditions, iron remains dissolved as ferrous Iron (Fe²⁺) and can be easily transported throughout irrigation systems. However, when water comes into contact with oxygen, often within irrigation systems, ferrous iron oxidizes into insoluble ferric Iron (Fe³⁺), which forms a red-brown precipitate. It is important to know and understand the water quality characteristics of irrigation water to adequately plan, or mitigate, for potential iron challenges.
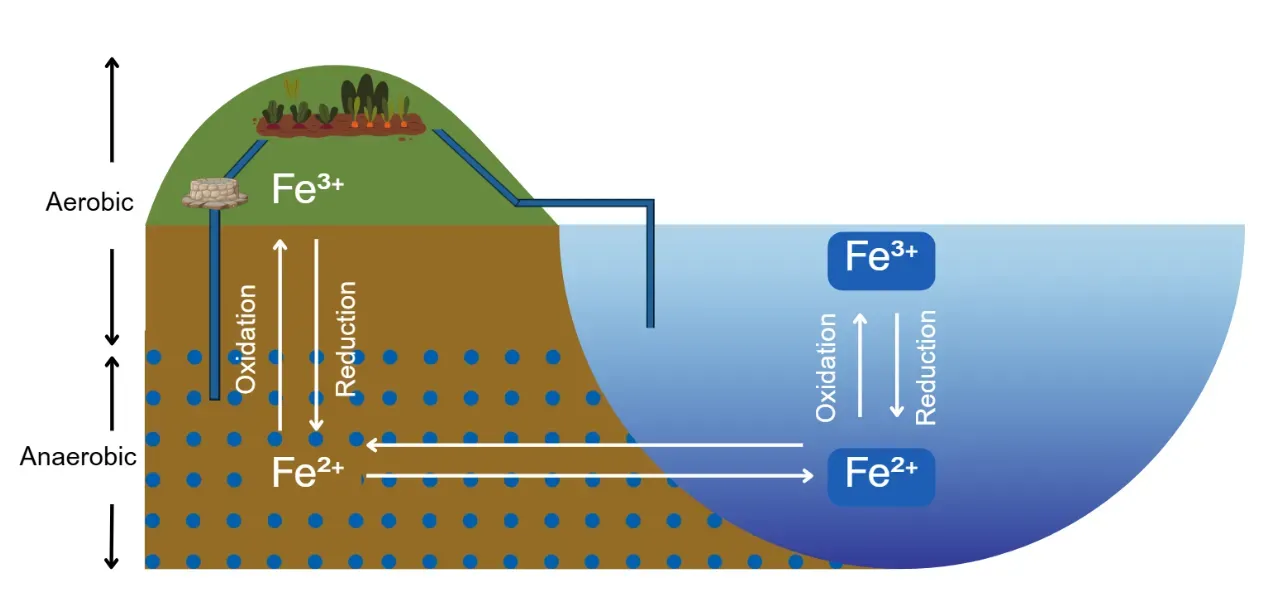
Figure 1. Simple illustration of Iron cycling in groundwater and surface water. In anaerobic conditions, insoluble ferric iron (Fe³⁺) may be reduced to soluble ferrous iron (Fe²⁺). In aerobic conditions, ferrous iron may be oxidized to ferric iron.
Environmental conditions impact the presence and form of iron in water. Soluble iron is more stable under anaerobic, acidic pH (acidic) conditions. As pH increases and becomes more alkaline, iron is more likely to precipitate. Excess nutrients in water, particularly nitrogen and phosphorus, fuel iron-reducing bacteria that facilitate the exchange of iron from soil to water. Nutrients may also enhance algae growth, which depletes oxygen levels and prevents iron from precipitating out of water before entering irrigation systems. Waterlogged or compacted soils with poor drainage can exhibit similar anaerobic conditions promoting iron solubility.
Microbial Drivers of Iron Cycling
Microbial activity plays a significant role in iron cycling and can be a nuisance in irrigation systems. In aerobic conditions in the presence of oxygen, iron-oxidizing bacteria use soluble iron as an energy source, producing biofilms or slime that are typically orange or brown. When this reaction occurs within an irrigation system, slimes can clog and damage irrigation infrastructure and are notoriously difficult to eliminate.
Conversely, iron-reducing bacteria thrive in anaerobic environments, converting insoluble iron found in soil and parent materials into soluble iron. This transformation increases soluble ferrous iron in groundwater and increases iron mobility in low oxygen environments.
Impacts of Iron in Irrigation Water
Iron precipitates and slimes can severely impact irrigation infrastructure by clogging drip lines and emitters and increasing the frequency of filter cleanings and damage. Iron precipitates can accumulate on metal components in aging irrigation infrastructure such as pond suction pipes and main or submain lines. As corrosion advances, flakes of iron may detach and be transported through the irrigation system, further contributing to clogging problems. These issues lead to decreased irrigation distribution uniformity, decreased water use efficiency, higher labor demands, and increased costs. Even relatively low iron concentrations, greater than 0.1 ppm, may cause drip system clogging, while levels above 0.3 ppm can cause rust stains and discoloration on plants irrigated by overhead sprinklers. Table 1 shows concentrations of various water quality parameters and their impact on microirrigation clogging.
Potential Problem | Units | Degree of Restriction on Use | ||
Physical parameter | None | Slight to Moderate | Severe | |
Suspended Solids | mg/l | < 50 | 50 – 100 | > 100 |
Chemical parameter | ||||
pH | < 7.0 | 7.0 – 8.0 | > 8.0 | |
Dissolved Solids | mg/l | < 500 | 500 – 2000 | > 2000 |
Manganese | mg/l | < 0.1 | 0.1 – 1.5 | > 1.5 |
Iron | mg/l | < 0.1 | 0.1 – 1.5 | > 1.5 |
Hydrogen Sulphide | mg/l | < 0.5 | 0.5 – 2.0 | > 2.0 |
Biological parameter | ||||
Bacterial populations | maximum number/ml | < 10,000 | 10,000 – 50,000 | > 50,000 |
Table 1: Influence of water quality on the potential for clogging problems in microirrigation drip irrigation (1 mg/L = 1 ppm) (Ayers et al., 1985).
Iron in Agricultural Crops
Iron is important to plant function playing a role in photosynthesis, respiration,and nitrogen metabolism among other processes. In general, iron is not toxic to plants unless present in high concentrations or in waterlogged, acidic soils. Irrigation water with iron concentrations exceeding 5 ppm (5 mg/L) may become toxic to plants, especially in acidic conditions with pH below 5.5. As soil and water pH decreases, iron becomes more soluble and bioavailable to plants, increasing the risk of toxicity. Iron toxicity in plants can cause stunted growth, reduced root development, and decreased chlorophyll content. Visual symptoms of iron toxicity are inconsistent but may include red/brown discoloration or spots, yellowing of leaves, and reduced size of plant. Plant tolerances to iron vary widely. For example, toxicity may occur in wheat when plant tissue iron concentrations >100 ppm, and in cherry plant tissue concentrations >1,300 ppm.
Iron deficiency in plants may occur in well aerated, alkaline conditions where iron precipitation is dominant and unavailable for plant uptake. Deficiencies may also occur when excess manganese is present, which acts as an oxidizing and converts iron to solid form in acidic conditions. Chlorosis, or yellowing of leaves, in younger leaves may be a visual indication of iron deficiency. Most plants obtain sufficient iron from neutral or acidic soils and deficiency thresholds depend on species. For grapevines, plant tissue analysis should show iron levels above 30 ppm to be considered sufficient.

Photo 1: Iron deficiency in grapevines causing chlorosis (photo credit: Peter Christensen).
Managing Iron in Irrigation Water
Water quality testing - The most critical step in managing iron in irrigation water is to perform water quality testing. Water samples should be collected from the water source and at various points throughout the irrigation system, such as at the ends of drip lines. Samples can then be sent to water testing laboratories for analysis. Confirming the presence and concentration of iron in addition to locating iron precipitation and clogging, provides necessary information for developing appropriate control methods.
Physical treatments - Understanding oxygen levels at various water depths in irrigation ponds can help inform pump placement. Adjusting the pump suction pipe to draw water from higher, oxygenated depths where iron precipitation has already occurred reduces soluble iron entering the irrigation system. This can be achieved by manually raising the pump or attaching a floating intake filter. Periodically clean intake filters to maintain pump efficiency.
Iron should be precipitated out of water before it enters irrigation systems. Physically aerating water allows for soluble iron to precipitate and settle out of the water before causing damage. Aerators or pond bubblers can be placed directly into ponds, reservoirs, or cisterns before water enters irrigation lines. For groundwater, an aeration and settling system can be implemented above ground after the pump but before water enters irrigation lines. Iron will precipitate out of the groundwater within the settling system, reducing the concentration of iron entering the irrigation system.
Adjusting water filtration systems based on water quality test results is recommended. Good water quality posing a low risk of clogging may only require a screen or disc filter, while lower quality water can require a sand media filter or multiple filtration systems. UCCE articles “How to Choose a Filter,” and “Filtration,” provide guidance on choosing appropriate filters for microirrigation systems. Additionally, regularly flushing drip lines removes mineral and biological buildup that causes clogging.
Chemical treatment - Chlorination is effective for oxidizing iron and killing iron-loving bacteria responsible for biofilm and slime formation. Inject chlorine before the filtration system to remove iron precipitate. For both iron precipitation and iron bacteria, inject chlorine to a residual of 1ppm free chlorine per liter at the end of the drip line. This is an adequate concentration to prevent iron clogging, kill bacteria, and remain safe water quality standards for plant health. Various other control methods exist such as acid injection to reduce pH and phosphonic acid. Both practices inhibit iron precipitation but must be used before any ferrous iron has precipitated to ferric iron. For more detailed information on chlorination and other chemical treatments, check out UCCE articles on “Iron, Manganese,” and “Chlorination.”
Soil Management - Iron toxicity in crops can be alleviated by implementing practices that decrease anaerobic conditions and promote soil aeration, infiltration, and iron precipitation. Cover crops, compost, gypsum, and in some cases tillage or drainage diversions may be appropriate to improve soil oxygen levels and move water off of fields. Under acidic soil conditions, iron toxicity may be managed by applying lime to soil. Lime can effectively raise the pH of soil creating alkaline conditions that favor iron precipitation.
It is difficult to amend for iron deficiency since iron is not mobile once in an oxygenated environment. Iron deficiency may be addressed in alkaline conditions by lowering soil pH with elemental sulfur, however this may take years to see results.
Monitoring and maintenance - Monitoring water quality and irrigation infrastructure can help mitigate iron problems before they cause severe damage. Promoting water oxygenation in irrigation ponds encourages iron precipitation and reduces the amount of soluble iron entering an irrigation system. Excess nutrients (e.g. Nitrogen and Phosphorus) reduces dissolved oxygen in water through eutrophication. Controlling nutrients from entering water sources can help maintain adequate oxygen levels.
Regularly inspecting irrigation pumps, filters, sprinklers, and lines can provide valuable information on the status and impact of iron management strategies. Iron precipitation may occur near leaks, tears, or cracks in irrigation lines and in emitters, sprinklers, and filters where water becomes oxygenated leaving behind a red/brown residue. Repair or replace damaged and aging parts. Red, brown, yellow, orange, or grey slime suggests the presence of iron bacteria.
Water Testing Labs
There are several reputable water testing labs that serve Northern California farmers and ranchers. For example:
A&L Western Laboratories, INC.
1311 Woodland Ave, Suite 1
Modesto, CA 95351
Phone: 209-529-4080
https://al-labs-west.com/water-analysis/
Alpha Analytical Laboratory in Ukiah, CA (Mendocino County) –
208 Mason St
Ukiah, CA 95482
Phone: 707.468.0401
ClientServices@alpha-labs.com
https://www.alpha-labs.com/services-listing
Alpha Analytical Laboratory in Petaluma, CA (Sonoma County) –
737 Southpoint Blvd
Suites C & D
Petaluma, CA 94954
Phone: 707.769.3128
Brelje & Race Laboratories in Santa Rosa, CA (Sonoma County)
425 South E Street
Santa Rosa, CA 95404
Phone: 707.544.8807
Dellavalle Laboratory Inc.
502 Mace Boulevard, #2-B
Davis, CA 95618
800-228-9896
client.services@dellavallelab.com
https://dellavallelab.com/agricultural-services/
More labs can be found at: https://ucanrvineyards.neocities.org/
Funding and Technical Support
Some programs offer financial or technical assistance for practices to improve water quality and irrigation system infrastructure that may be helpful for iron management:
- NRCS EQIP – Funding for water conservation and water use efficiency projects which may address water quality concerns.
- SWEEP – California’s State Water Efficiency and Enhancement Program supports water use efficiency upgrades such as filtration and pumps.
- DWR Grants – Watch for new funding opportunities around water use efficiency, water supply and management, and water quality.
Questions or need help diagnosing iron issues in your irrigation system?
Contact your local UCCE Advisor for assistance
Resources
- Ayers, R. S., & Westcot, D. W. (1985). Water quality for agriculture. FAO. https://www.fao.org/3/t0234e/t0234e00.htm
- Chorover, J., M. K. Amistadi, et al. Biogeochemical Weathering of Serpentinites: An Examination of Incipient Dissolution Affecting Serpentine Soil Formation. Applied Geochemistry. https://www.sciencedirect.com/science/article/pii/S0883292715000098?via%3Dihub
- County of Napa. Groundwater Trends and Conditions. https://www.countyofnapa.org/3089/Groundwater-Trends-and-Conditions
- Emerson, D., E. J. Fleming, and J. M. McBeth. Iron-Oxidizing Bacteria: An Environmental and Genomic Perspective. Frontiers in Microbiology. https://www.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2012.00096/full
- Government of South Australia. Best Management Practices for Clogging of Irrigation Infrastructure by Iron and Sulphate Bacteria. South Australia Department of Primary Industries and Regions. Accessed August 6, 2025. https://limestonecoastwine.com.au/wp-content/uploads/2017/07/Iron-and-Sulphate-factsheet-FINAL.pdf
- Harish, V., S. Aslam, S. Chouhan, Y. Pratap, and S. Lalotra. Iron Toxicity in Plants: A Review. Department of Agronomy, School of Agriculture, Lovely Professional University, Phagwara, Punjab, India.
- Kansas State University Research and Extension. Soil Amendments for Iron Deficient Trees. https://blogs.k-state.edu/wildwestdistrict/2024/04/05/soil-amendments-for-iron-deficient-trees/
- Lovley, Derek R., and Elizabeth J. P. Phillips. “Organic Matter Mineralization with Reduction of Ferric Iron in Anaerobic Sediments.” Applied and Environmental Microbiology 51, no. 4 (1986): 683–689. https://doi.org/10.1128/aem.51.4.683-689.1986
- Oak Ridge National Laboratory. Ecological Soil Screening Levels for Iron. https://rais.ornl.gov/documents/eco-ssl_iron.pdf
- Rutgers Cooperative Extension. Management of Iron in Irrigation Water. Accessed August 6, 2025. https://njaes.rutgers.edu/fs516/#:~:text=It%20is%20recommended%20to%20inject,the%20pH%20of%20the%20water
- Singh, R., and R. Sharma. Biogeochemistry of Iron Enrichment in Groundwater: An Indicator of Environmental Pollution and Its Management. https://www.mdpi.com/2071-1050/14/12/7059
- U.S. Forest Service. Serpentine Soils and Plant Adaptations. https://www.fs.usda.gov/wildflowers/beauty/serpentines/adaptations.shtml
- U.S. Geological Survey. The Occurrence of Ground Water in the United States, with a Discussion of Principles. Water-Supply Paper 1459-A. https://pubs.usgs.gov/wsp/1459a/report.pdf
- U.S. Geological Survey. Water Quality in Principal Aquifers of the United States, 1991–2010. https://pubs.usgs.gov/fs/2014/3114/pdf/fs2014-3114.pdf
- U.S. Geological Survey. Water Quality in Principal Aquifers of the United States, 1991–2010. https://pubs.usgs.gov/fs/2010/3060/
- University of California Agriculture and Natural Resources (UCANR). Maintenance of Microirrigation Systems: Biological Clogging. Accessed August 6, 2025. https://ucanr.edu/site/maintenance-microirrigation-systems/biological-clogging-slimes-algae-etc
- University of California Agriculture and Natural Resources (UCANR). Maintenance of Microirrigation Systems: How to Choose a Filter. Accessed August 6, 2025. https://ucanr.edu/site/maintenance-microirrigation-systems/how-choose-filter-1
- University of California Agriculture and Natural Resources (UCANR). Maintenance of Microirrigation Systems: Iron and Manganese in Microirrigation Systems. Accessed August 6, 2025. https://ucanr.edu/site/maintenance-microirrigation-systems/iron-manganese
- University of California Agriculture and Natural Resources. Diagnosing and Correcting Iron Deficiency in Landscape Trees. https://ucanr.edu/sites/default/files/2018-07/287755.pdf
- University of Florida IFAS Extension. Unclog Drip Emitters in Your Greenhouse. https://www.greenhousegrower.com/production/unclog-drip-emitters-in-your-greenhouse
